Blood Products and Services Information
This service allows prospective patients who are to undergo elective surgery or have chronic red blood cell transfusion requirements to recruit family and friends with compatible blood groups to donate blood on their behalf. This request must be made by the patient’s clinician through formal referral (see a link to the referral documents below), and the decision to donate must be entirely voluntary.
Designated single-donor platelet donations can also be requested when HLA-matched platelet products are needed. This request typically arises when there is suspicion of platelet antibodies in patients whose platelet counts fail to increment following transfusion, and other causes have been excluded (e.g., fever, infection, or splenomegaly). The patient’s clinician must request HLA-typing of their patient via their own tissue immunology laboratory, and these results are sent to the South African Bone Marrow Registry for matching with a donor. The donor’s contact details are then forwarded to the WCBS Apheresis Unit, which will try to locate them to establish if they are eligible and willing to donate. The procedure involves placing the donor on an apheresis machine for approximately 90 minutes.
Designated donors must comply with the general blood donor acceptance criteria and undergo mandatory donation testing. However, TPHA-reactive or cross-reactive donations may be issued with permission from the WCBS CEO/Medical Director, in consultation with the patient’s clinician.
All designated blood donations from first- and second-degree relatives must be irradiated to prevent transfusion-associated graft-versus-host disease, which is related to the potential sharing of HLA-haplotypes between the donor and recipient.
Women of childbearing age shouldn’t receive blood from their spouse or his relatives, as this could lead to a blood group incompatibility that can affect the safety of future pregnancies.
Download clinician’s request for specialised donations for red cell and plasma designated donations.
Download patient’s consent for specialised donations for red cell and plasma designated donations.
For red cell and plasma designated donation enquiries, please contact Sr Tania Paarman, Manager – Specialised Donations.
Sr Tania Paarman: Telephone +27 (0)21 507 6393 | Email Tania@wcbs.org.za
For HLA-matched platelet designated donation enquiries, please contact Kay Abrahams, Manager – Apheresis Donations.
Kay Abrahams: Telephone +27 (0)21 507 6395 | Email Kay@wcbs.org.za
Therapeutic phlebotomy refers to the practice of donating blood to assist with the management of medical conditions that result in iron overload or high red cell production. Common causes for these conditions include hereditary haemochromatosis, secondary polycythaemia from smoking or testosterone use, and polycythaemia vera.
We usually use blood donated by therapeutic donors after their first phlebotomy, provided that they do not have a primary polycythaemia (e.g., polycythaemia vera, high-affinity haemoglobin, or familial polycythaemia) and meet regular donor acceptance criteria.
Patients who are advised to donate blood for medical reasons and require more frequent blood donations than a regular blood donor (i.e., more often than every two months) or who do not meet routine acceptance criteria (e.g. warfarin use) must be referred by their clinician through a formal referral process (see the link to the referral documents below). The referral information will be reviewed by the WCBS Head of the Medical Division to determine whether it is safe for phlebotomy to take place at a non-medical facility and if the blood is suitable for transfusion to a patient. Frail patients or patients with significant cardiovascular comorbidity are advised to undergo therapeutic phlebotomies in hospital settings where there is medical support.
The blood from the first therapeutic phlebotomy procedure is not used for transfusion purposes, as per international guidelines for blood donation. A charge will apply for the first phlebotomy unless the donor is already a registered donor at WCBS or SANBS with a history of donating valid units, and any subsequent phlebotomies where the blood must be discarded due to the underlying diagnosis or other deferral criteria.
We accept new therapeutic donors between the ages of 16 and 75 years. Elderly donors are required to obtain regular permission from their doctors to continue donating blood. Please note that it is the responsibility of the referring doctor to prescribe the donation intervals for their patient.
Download registration forms:
For therapeutic phlebotomy enquiries, please contact Specialised Donations.
Telephone +27 (0)21 507 6320/6397/6393 | Email phlebotomy@wcbs.org.za
The Western Cape Blood Service (WCBS) provides more than 100 emergency blood banks, stocked with variable quantities of O RhD negative and O RhD positive emergency blood, for use in life-threatening emergencies when there is no time to wait for crossmatched blood from a Blood Bank. The banks are situated at hospitals and healthcare facilities that do not have a Blood Bank onsite; exceptions apply.
Group O RhD negative emergency blood is a particularly scarce resource and is known to be used irresponsibly. This product should strictly be used for RhD negative patients who urgently require red cell transfusions. In times of critical shortage, the restrictions should extend to only transfusing RhD negative women of child-bearing age with O RhD negative emergency blood. The risk of transfusing RhD negative patients with RhD positive red cells relates to the potential development of RhD antibodies that could affect future pregnancies and transfusion with RhD positive blood. Interestingly, up to 15% of RhD negative people show no immunogenic response to RhD positive transfusions and immunosuppressed patients are likely to demonstrate a muted or absent response.
If the RhD status of the patient is unknown, a RhD slide test should first be performed. The test is quick and results are available within 15 – 60 seconds.
It is important to note that patients are more likely to be RhD positive as the rate in the South African population is approximately 95%.
Crossmatched blood is the safest product for the patient, but there are urgent situations where there may not be sufficient time to wait.
Ideally, no more than 4 units of group O emergency blood should be transfused, due to potential incompatibility issues of non-ABO red cell antigens. If the clinician anticipate that the patient may need more blood, it would be best to transfer them to a hospital that has quicker access to a Blood Bank, to receive crossmatched blood.
In the event that only O RhD positive blood may be available for a RhD negative patient, it is advisable to administer anti-D immunoglobulin at a dose of 125 IU (25 µg) for each 1ml of red cells transfused. If anti-D immunoglobulin was not administered, the patient must be counselled regarding risk to future Rh incompatible pregnancy.
The emergency blood stock is replenished by WCBS as soon as it is used, and on notification from the hospital/healthcare facility. Unused emergency blood stock is replenished at approximately 3-weekly intervals.
We encourage clinicians to adhere to these guidelines to ensure that O RhD negative emergency blood is reserved for patients who need it most.
For emergency blood enquiries please contact Nawaal Gamieldien, WCBS Emergency Blood Bank Co-ordinator.
Nawaal Gamieldien: Telephone +27 (0)21 507 6476 | Cell +27 (0)83 564 9297 | Email Nawaal@wcbs.org.za
Red cell components are useful for their iron-rich stores and oxygen-carrying capacity. Red cell transfusions are indicated for a wide range of medical and surgical conditions resulting in acute or chronic anaemia.
Buffy-coat Depleted Red Cell Concentrate
Volume: 250 ml to 350 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Leucocyte count: ≤2.4 x 109/unit
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 42 days
Pre-storage Leucocyte Depleted Red Cell Concentrate
Volume: 210 ml to 310 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Leucocyte count: <1 x 106/unit
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 42 days
Leucocyte Depleted Red Cell Concentrate (filtered in the Blood Bank)
Volume: 210 ml to 310 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Leucocyte count: ≤5 x 106/unit
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 24 hours
Washed Leucocyte Depleted Red Cell Concentrate in Albumin/Saline Additive
Volume: 320ml to 420ml
Haematocrit: n/a
Anticoagulant: CPD
Additive solution: n/a
Washing solution: Sodium Chloride BP 0.9%
Additional solution: 20% Albumin plus BP 0.9% Sodium Chloride
Storage temperature: 2 °C to 6 °C
Shelf life: 24 hours after processing
Whole blood products are rarely indicated for use in massive haemorrhage and neonatal exchange transfusions. Red cell components are more appropriate for use in situations where oxygen-carrying capacity requires boosting.
Whole Blood
Volume: 468 ml to 558 ml
Anticoagulant: CPD
Storage temperature: 2 °C to 6 °C
Shelf life: 21 days
Whole Blood Leucocyte Depleted
Volume: 425 ml to 525 ml
Leucocyte count: ≤ 5 x 106/unit
Anticoagulant: CPD
Storage temperature: 2 °C to 6 °C
Shelf life: 24 hours if prepared in the Blood Bank and 21 days if prepared in Components Processing
All infant and paediatric blood products are leucocyte-depleted to reduce the risk of alloimmunisation, immune-mediated adverse transfusion reactions and disease transmission.
Infant Red Cell Concentrate
Volume: 35 ml to 75 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 42 days
Paediatric Red Cell Concentrate
Volume: 90 ml to 150 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 42 days
Haemoconcentrate
Product code: LEURHL
Volume: n/a
Haematocrit: ≥ 80 %
Red cell recovery: ± 80 %
White cell removal: > 95 %
Red cell concentrate: ≤ 72 hours
Anticoagulant: CPD
Additive solution: SAGM
Storage temperature: 2 °C to 6 °C
Shelf life: 24 hours after processing
Washed Pre-storage Leucocyte Depleted Red Cell Concentrate
Product code: LRWRBC
Volume: > 185 ml
Haematocrit: 0.5 l/l to 0.7 l/l
Total protein content: < 5 mg/unit
Anticoagulant: CPD
Additive solution: SAGM
Washing solution: Sodium chloride solution B.P. 0,9 %
Storage temperature: 2 °C to 10 °C
Shelf life: 24 hours
Paediatric Single Donor Platelet
Volume: 100 ml to 200 ml
Platelet count: ≥ 1.0 to 2.3 x 1011/l
Anticoagulant: ACD-A
Storage temperature: 20 °C to 24 °C
Shelf life: 5 days
Infant Single Donor Platelet
Volume: 40 ml to 60 ml
Platelet count: 0.5 to 0.9 x 1011/l
Anticoagulant: ACD-A
Storage temperature: 20 °C to 24 °C
Shelf life: 5 days
Paediatric Fresh Frozen Plasma
Volume: 100 ml to 160 ml
FVIII: C ≥0.7 IU/ml
Anticoagulant: CPD
Storage temperature: Below minus 18 °C
Shelf life: 6 hours after removal from storage
Paediatric Fresh Frozen Plasma Leucocyte Depleted
Volume: 100 ml to 160 ml
Anticoagulant: CPD
Storage temperature: ≤24 °C
Shelf life: 6 hours after removal from storage
Fresh Frozen Plasma Low Titre Anti-T
Volume: 210 ml to 350 ml
FVIII:C: ≥0.7 IU/ml
Anticoagulant: CPD
Storage temperature: Below minus 18 °C
Shelf life: 6 hours after removal from storage
Cryoprecipitate Low Titre Anti-T
Volume: 9 ml to 11 ml
FVIII:C: ≥80 IU/unit
Fibrinogen: ≥12 mg/ml
Anticoagulant: CPD
Storage temperature: Below minus 18 °C
Shelf life: 4 hours after removal from storage
Paediatric Whole Blood
Volume: 140 ml to 200 ml
Anticoagulant: CPD
Storage temperature: 2 °C to 6 °C
Shelf life: 21 days
Platelets are responsible for cessation of bleeding at the sites of endothelial injury. Platelet transfusions are indicated for the prevention or management of active bleeding as a result of reduced platelet numbers (thrombocytopenia) or abnormalities of platelet function. The three different products currently available are pooled platelets, leucocyte reduced (filtered) platelets, and single donor (apheresis) platelets. All three platelet products are regarded as equivalent in terms of post-transfusion increments and haemostatic efficacy. The pooled products carry a higher risk of alloimmunisation (i.e. antibody formation to donor platelet antigens), and therefore single donor or leucocyte reduced (filtered) pooled platelet products are recommended for patients with chronic transfusion needs (e.g. those with haematological malignancies or undergoing stem-cell transplants). Studies have shown that a leucocyte poor platelet pool is equivalent in terms of platelet alloimmunisation compared to a single donor (apheresis) platelet.
Leucocyte Depleted Pooled Random Donor Platelet
Volume: ≥ 200 ml
Platelet count: ≥ 2.4 x 1011/unit
Leucocyte count: ≤ 5 x 106/unit
Anticoagulant: CPD
Storage temperature: 20 ºC to 24 ºC
Shelf life: 5 days if filtered in the Processing Laboratory. 6 hours if filtered in the Blood Bank Laboratory.
Pooled Random Donor Platelet
Volume: ≥ 200 ml
Number of donations in the pool: ≥4
Platelet count (for a pool of ≥4 units): ≥ 2.4 x 1011/unit
pH (within 24 hours of expiry): ≥ 6.4 at 22 ºC to 24 ºC
Anticoagulant: CPD
Storage temperature: 20 ºC to 24 ºC
Shelf life: 5 days
Single Donor Platelet
Volume: ≥ 200 ml
Platelet count: ≥ 2.4 x 1011/unit
Leucocyte count: ≤ 5 x 106/unit
Anticoagulant: ACD-A
Storage temperature: 20 ºC to 24 ºC
Shelf life: 5 days
Plasma products are derived by the centrifugation of anticoagulated whole blood within 18 hours of donation. These products are typically prescribed for the replacement of coagulation proteins (or clotting factors). Fresh frozen plasma (FFP) contains all the clotting factors at normal physiological levels and can be leucocyte-depleted to reduce the risk of immune-mediated adverse transfusion reactions. Cryoprecipitate is formed by the thawing of FFP and precipitation of the product that is rich in Factor VIII, Factor XIII and fibrinogen.
Fresh Frozen Plasma
Volume: 210 ml to 350 ml
FVIII:C: ≥0.7 IU/ml
Anticoagulant: CPD
Storage temperature: Below minus 18 ºC
Shelf life: 6 hours after removal from storage
Leucocyte Depleted Fresh Frozen Plasma
Volume: 210 ml to 350 ml
Anticoagulant: CPD
Storage temperature: ≤ 24 ºC
Shelf life: 6 hours from time of removal from storage
Cryo Poor Fresh Frozen Plasma (Cryosupernatant)
Volume: 200 ml to 340 ml
Anticoagulant: CPD
Storage temperature: Below minus 18 ºC
Shelf life: 6 hours after removal from storage
Cryoprecipitate (Pooled)
Volume: ±50ml
FVIII:C: ≥80 IU/unit in the pool
Fibrinogen: ≥12 mg/ml per unit in the pool
Anticoagulant: CPD
Storage temperature: Minus 18 ºC or below
Shelf life: 4 hours after removal from storage
WCBS provide irradiated blood products, on request from the clinician.
Blood irradiation results in the eradication of donor T-lymphocytes in the blood product, for the purpose of prevention of transfusion associated graft versus host disease. For more information about the indications and benefits of blood irradiation, please refer to the Clinical Guidelines for the Use of Blood and Blood Products in South Africa, 6th Edition.
Please note that there is an additional cost for the irradiation procedure.
Blood irradiation is performed at the Groote Schuur Blood Bank and Tygerberg Blood Bank, thus, timeous ordering is required.
For more information, contact your closest Blood Bank.
Babies may require multiple red cell concentrate infusions for the management of prolonged anaemia. Through the Limited Donor Exposure Programme (LDEP), doctors can reserve a minimum of two up to a maximum of four units of single donor infant red cell concentrates for their patient. The advantages of LDEP include reduction in donor exposure for the baby to limit the risk of red cell alloimmunisation and infection transmission.
The clinician must clearly indicate “LDEP” on the cross-match laboratory request and the number of units to be reserved. If the quantity is unspecified, the Blood Bank will reserve two units for the patient. A new Blood Bank sample is required if the previous unit of blood or blood product was issued more than forty-eight hours before.
Neonates who possess irregular red cell antibodies or have strongly positive crypt antigen tests may be excluded from the LDEP programme – please discuss these cases on an individual basis with the Blood Bank staff.
For more information, contact your closest Blood Bank.
A core responsibility of the Western Cape Blood Service (WCBS) is to continue providing a safe and sustainable blood supply for patients who require blood transfusions.
WCBS will consider requests for blood for non-clinical use from tertiary training institutions, affiliated organisations, life science companies, assay development companies, and diagnostic testing laboratories.
Requests may include the following types of blood products (and samples) that are not suitable for transfusion purposes:
- Blood that is reactive for a transfusion transmissible infection tested by WCBS, for example, HIV positive plasma, hepatitis B-positive plasma, hepatitis C-positive plasma and syphilis positive plasma.
- By-products from blood component processing, for example, buffy coats that are not used in the production of platelet products (rich in platelets and white blood cells).
- Expired blood, for example, warm returned red cell concentrates that were issued for a patient and returned unused outside the storage temperature specifications.
- Non-viable blood, for example, an underweight blood pack.
- Blood donor sample tubes, drawn from the sample diversion pouch of the blood collection bag.
- Volunteer sample tubes drawn directly from the arm, where no unit of blood is collected.
- In-date blood products from a specified blood group may be considered only if excess blood is available and the blood is not needed for a patient(s).
- WCBS may also be asked to assist with collecting blood from a research volunteer for purposes of their own research project or another research project.
Blood donors at WCBS give consent for the use of their blood for non-clinical use. The confidential donor questionnaire states: “I consent to samples of my blood and/or donation data being used anonymously for scientific research aimed at improving the safety of the blood supply and donor health, and that on occasion WCBS may permit researchers to request additional samples from me with my specific consent”. Donor consent is implied in the WCBS privacy statement.
WCBS provides donor samples to third parties – the samples are strictly de-identified and anonymised. All blood issued for non-clinical use must have undergone mandatory donation testing before being issued. WCBS does not provide patient samples to third parties.
Where consent may be required but falls outside the scope of the statement above, the requester will need to provide a separate donor consent form.
Requests should be addressed on a letterhead to Hayley Alie, Hospital Liaison Officer (hayleya@wcbs.org.za) and include the following particulars and a supporting document:
- Name and contact details of researcher/requester
- Project title and research institution’s ethics approval reference number
- Type of blood product or sample required (e.g. expired red cell concentrate, buffy coat, hepatitis B-positive plasma)
- Quantity and frequency of required blood product or sample (e.g. 200ml) or number of packs/samples and dates required
- Purpose for which the blood product or sample is required (e.g. research purposes, population reference ranges, instrument evaluation and reagents purposes).
- Evidence of research institution’s ethics committee approval
- Process to ensure safe disposal of blood packs or sample tubes
WCBS will gladly review the request and reply to the researcher/requester.
The researcher/requester will need to provide annual request renewals and annual ethics approvals to enable WCBS to continue supplying blood products and samples for the research project.
For more information, contact Hayley Alie, Hospital Liaison Officer (hayleya@wcbs.org.za)
Blood Bank Turnaround Times
The below turnaround times (TATs) exclude transportation. If you wish to discuss the TATs, kindly contact your nearest Blood Bank.
| Services | Turnaround Times |
|---|---|
| STAT Crossmatch | 20 – 30 minutes |
| Full Crossmatch | 2 hours |
| Routine Crossmatch | 2 hours or for when required |
| Group and Screen | 2 hours |
| Red Cell Products with Repeat Electronic Crossmatch | 10 minutes if repeat order |
| Crossmatch with Positive Antibody Screen | 2 hours to 7 days depending on complexity of the antibody |
| Red Cell Products | 2 hours |
| Washed Red Cell Products | 6 – 9 hours |
| Haemoconcentrate | Notify the day before by 13:00 (24 -36 hours) – not available on Mondays |
| Single Donor Platelets | 3 – 11 hours depending on if there are emergency platelets available or if it has to be bled |
| Random Donor Platelets | 2 hours |
| Fresh Frozen Plasma | 2 hours ( 30 minutes if a repeat order) |
| Antibody Titration | 2 hours |
| Cryoprecipitate | 2 hours (30 minutes if a repeat order) |
| Irradiation of Blood Products | 2 hours depending on products |
| Group O Emergency Blood Stock Replenishment | 10 minutes |
| Designated Donation Blood Products | 2 days |
| Direct Coombs | 2 hours |
| Coombs Auto | 2 hours |
| Crypt Antigen Test | 2 hours |
| Anti-T Titration | 2 hours |
| Cord & Maternal (includes NaOH test) | 2 hours |
| Blood request for peripheral stem cell transplant recipient | 3 days, with the exception of HLA-matched platelets |
Instructions For Patient Samples
- The Blood Bank will only accept 6 ml EDTA samples (in emergencies, 4 ml EDTA samples may be accepted).
- The sample should not be taken from an intravenous drip site.
- Before collection, verify the patient’s identity by asking the patient to state his/her full name and date of birth. Compare this information with the request form and the patient’s printed label.
- Label the sample container before performing collection, at the patient’s bedside.
- All samples must be labelled with:
- Patient’s first and last names
- Patient’s hospital number
- Emergency number/identity number/D.O.B. can be substituted for folder number if not available
- If the sample cannot be sent to the Blood Bank immediately, it can be stored between 2 – 6 °C and delivered to the Blood Bank within 24 hours of collection.
- The sample must be transported in a leak-proof container (e.g. plastic pouch/bag), and the sample and request form must be stored separately.
- The ordering clinician’s MP number and the patient’s diagnosis should also appear on the request form.
- Label the sample tubes by placing the label in the correct position on the sample container: See diagram below.
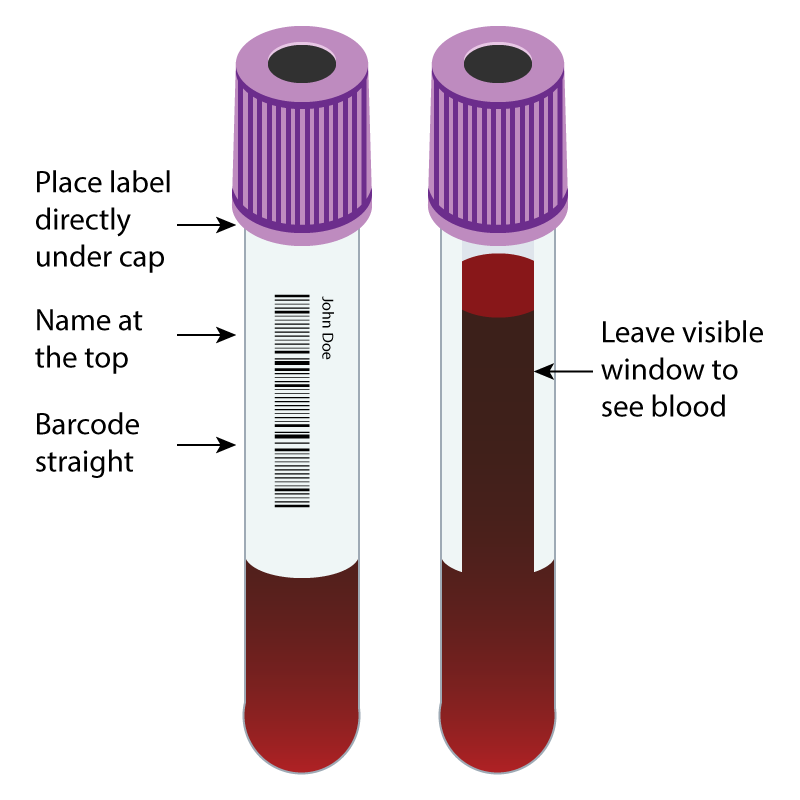
The Blood Bank will reject a sample and form that is received with any of the following issues:
- No patient identification
- Patient details on the form and the sample do not correspond
- Incorrect or expired sample tube
- No date, time or signature of the phlebotomist collecting the sample on the patient request form
- Insufficient volume to test the sample
The following information is required on the request form for billing purposes:
- Medical aid name and number (if applicable)
- Patient details, address and contact numbers (landline and cell phone)
- Patient email address
- Doctor’s name and practice number
